Being exposed to human immunodeficiency virus (HIV) can be a frightening experience, and testing to determine one’s HIV status can be even more worrisome. Though HIV poses many health complications, it also comes with a heavy stigma that may discourage many individuals from getting tested for the virus in a public setting. Additionally, HIV testing can be expensive and inaccessible to many individuals—presenting a need for an easier and more attainable testing method. In 2012, the OraQuick In-Home HIV Test was released into stores and made available for purchase to individuals over the age of 17 years, allowing people to test themselves for HIV in the comfort and ease of their own homes.1
Table of Contents
What is OraQuick?
OraQuick is the first and only FDA-approved at-home HIV test in the United States. After a long period of controversy concerning the morality of individuals having access to at-home HIV tests, the FDA finally approved OraQuick in 2012.2 Its approval demonstrates that progress has been made towards the gradual normalization of a once highly stigmatized virus. Dealing with the hassle and potential public stigma of visiting a health professional is no longer the only option available to individuals, thereby creating accessibility that could encourage more people to get tested for HIV. OraQuick is unique because it is fast and inexpensive, and it allows individuals to take the test in a private setting. This device is available to people all across the United States at pharmacies, grocery stores, and online retailers; however, international availability is limited.3
How it works

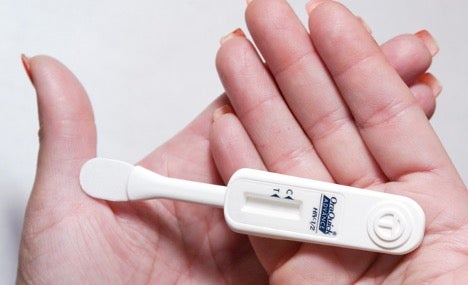
OraQuick works via mouth swab, and results become available within 20 to 40 minutes.4 The product functions by detecting HIV antibodies in saliva samples collected from the gum line. Because HIV antibodies take up to 3 months to develop after infection occurs, OraQuick users must meet the 3 month time frame for the most accurate results. To use the test, a person simply swipes the swab along their gumline and then inserts it into the test tube provided. Reading the test is simple. The test has a labeled “C” mark and a labeled “T” mark directly below it. After swiping the gumline, there should always be a line next to the “C” in a test; no “C” line indicates a malfunctional test. A line next to the “C” mark and no line next to the “T” mark indicates a negative test result. If there are two lines (dark or faint) next to both the “C” and “T,” the test result is positive. Following a positive test result, individuals should consult with a medical professional to verify their positive HIV status and, if applicable, determine a treatment plan.5
Before using OraQuick, it is recommended by the manufacturer that a few precautions are taken. First, the individual should not drink, eat, or use any oral products up to 30 minutes before taking the test, as any substance in the mouth has the potential to impair results. Dental products that may block access to the gums are also discouraged. Next, individuals should keep a timer close by to properly time the waiting period before proper results are shown. Many individuals may find comfort in companionship during this test by having a friend or a phone nearby for support, as many may find the waiting period for results to be stressful.5
Effectiveness
Although OraQuick is revolutionary in its efforts to promote and normalize HIV testing, the test itself is not always 100% accurate. Since oral fluid has lower levels of antibodies than blood, oral fluid tests are less accurate at testing for more recent exposure to HIV.4 Despite this drawback, OraQuick remains a very effective way to detect HIV, as researchers have found the home test to be accurate 99.98% of the time for those who do not have the virus and over 92% accurate for those who do have the virus.2 As a result of this margin of error, it is highly recommended that individuals receive secondary confirmation from a health professional after receiving a positive result. With that being said, OraQuick remains a useful public health asset in helping to prevent the transmission of HIV since it is estimated that nearly 20% of the 1.2 million infected Americans do not know they have the disease. 2 It is important to discover whether or not an individual is HIV-positive because the sooner the diagnosis is discovered, the sooner that person can start receiving treatment.
Pros and Cons
One benefit of OraQuick is the accessibility of the test. Not only can individuals purchase the test from local drugstores or with a simple computer click, they can also take the test in the privacy of their own home. Additionally, the test is priced around $38 from the manufacturer itself, which may be a low cost in comparison to taking a test at a clinic.5
The take-home test does come with some downsides. As previously mentioned, individuals must wait at least three months after potential HIV exposure before using OraQuick, as the test relies on the development and detection of HIV antibodies (it can take up to three months for HIV antibodies to develop). Further, the test is not a guaranteed positive. Following a positive test result, individuals must follow up with a licensed physician to verify the findings of the test and potentially start treatment. Lastly, individuals who are under the age of 17 years will not be able to purchase OraQuick, as a government-issued ID is required to legally buy the test.5
Concluding Remarks
OraQuick is a major step towards normalizing the practice of HIV testing by providing an accessible and simple testing method. As the test and its abundance continues to improve, the prevention of HIV transmission and the development of treatment methods will hopefully improve as well. Ultimately, education and awareness of safe sex practices remain some of the greatest assets in helping to combat HIV transmission. Regardless of results, people around the world have discovered several measures to prevent HIV transmission along with treatments for those who have been infected, and the disease continues to become increasingly manageable as we learn more.
References
1. Food and Drug Administration. Facts About In-Home HIV Testing. U.S. Food and Drug Administration. Web. 25 June 2020.
2. McNeil, Jr. Donald. “Rapid H.I.V. Home Test Wins Federal Approval” NY Times. Health, 3 July 2015.Web. 10 October 2016.
3. Shattner, Elaine. “This At-Home HIV Test Looks Simple, but Is It Accurate?” The Atlantic. Atlantic Media Company, 9 July 2012. Web. 11 Oct. 2016.
4. “Home Tests.” Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 16 Oct. 2015. Web. 11 Oct. 2016.
5. OraQuick. Why OraQuick. Web. 2022
Last updated: 3 March 2022.
